Molecules that detect, monitor, and help to treat disease
 On the third floor of Skirkanich Hall, Andrew Tsourkas is creating new magnetic nanoparticles that may revolutionize the detection of early cancer cells. By “early,” he means when molecular changes in the disease are occurring, but before any anatomical changes are visible.
On the third floor of Skirkanich Hall, Andrew Tsourkas is creating new magnetic nanoparticles that may revolutionize the detection of early cancer cells. By “early,” he means when molecular changes in the disease are occurring, but before any anatomical changes are visible.
Detecting Cancer with Nanoparticles
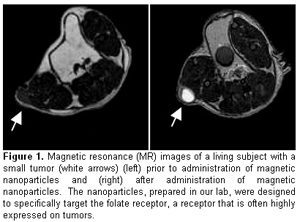
Today’s magnetic resonance imaging scans typically only reveal tumors that alter the normal anatomy – appearing as a lump or nodule on a tissue. However, molecular changes unique to cancer cells are present at much earlier stages of the disease. Tsourkas fabricates nanoparticles—about one hundred times smaller than the width of a single red blood cell—that can specifically find these cancer cells based on their molecular changes. The particles then report the location of the cancers cells by eliciting a bright signal on MRI. The ability to detect cancer at very early stages could vastly improve the effectiveness of current therapies since the disease will have had less time to spread. Tsourkas believes that early detection is the key to improving the rate of patient survival.
Nanoparticles and the Mystery of Back Pain
By changing the formulation of the magnetic nanoparticles, Tsourkas has also created tools that can help locate sites of back pain. Beth Winkelstein, who studies pain and injury biomechanics, is collaborating with Tsourkas to solve and help manage the mystery of back pain of unknown origin. Winkelstein hypothesizes that in many cases back pain may result from a transient injury that causes inflammation, but does not result in any structural damage. This could explain why a clinician may not see anything using conventional imaging approaches. If there were some way to track the inflammation, then managing a patient’s pain might be more effective.
Tsourkas has developed nanoparticles that target macrophages—the large white blood cells that “eat” foreign particles and infectious microorganisms. Macrophages flock to inflammation like moths to a flame. Tsourkas’s nanoparticles target the macrophages, labeling them along the nerve root so that they can be seen by MRI. In effect, they locate the inflammation.
“You’ll get a quantitative measure of inflammation,” Tsourkas says of his novel technique. “Maybe it will give us a better measure of the extent of pain.” Although the cause of pain might remain unknown, the nanoparticle could potentially be used to “monitor the effects of anti-inflammatory therapies, and gauge how to adjust treatment,” he adds.
Nanoparticles and Personalized Patient Care
Future applications for nanoparticles are bound to grow. To treat lupus, an autoimmune disease, for example, physicians are attempting to deplete the body’s B cells—cells that usually are protective but in autoimmune diseases produce antibodies that attack the body’s own cells. Rheumatologist Bob Eisenberg, another of Tsourkas’s collaborators, tests the blood of lupus patients to see how well the depletion treatment is working. He has found, however, that patients can still relapse even with a low B-cell blood count. Apparently, testing the blood for B cells doesn’t seem to be a very accurate reflection of the number of B cells in the patient’s entire body.
That’s where engineered imaging nanoparticles come into play. Collaborating with Eisenberg, Tsourkas is designing nanoparticles that will find B cells wherever they are in the body, not just in the bloodstream. For example, Tsourkas’s extremely tiny agents are able to seek out B cells in organs such as the spleen and lymph nodes.
“Maybe,” Tsourkas says, “it’s possible that although the B cells in the blood are low, much higher levels remain in the spleen. Perhaps, the depletion of B cells in the spleen will help a clinician to better decide when additional treatment is needed, before a relapse occurs. Each patient would be treated according to his or her own specific needs … truly personalized patient care.”
